![]() During the last decades, sufficiently activated group 4 metallocenes and related complexes have emerged as highly active and versatile catalysts for the homogeneous Ziegler-Natta-type polymerisation of ethylene and higher α-olefins. One of the most intriguing advancements in this field was the introduction of ansa-metallocenes or metallocenophanes which contain a bridging moiety between the two η5-coordinated fragments. These have allowed for the synthesis of a vast range of novel stereo regular polyolefins. Another great success was the development of related Group 4 constrained geometry complexes (CGCs) where one of the η5-coordinated ring systems of a metallocenophane is formally replaced by an η1-coordinated amido moiety. Due to their more accessible active site, these compounds display superior characteristics in the copolymerisation of ethylene and higher α-olefins, resulting in another class of polyolefins with exceptional features.
During the last decades, sufficiently activated group 4 metallocenes and related complexes have emerged as highly active and versatile catalysts for the homogeneous Ziegler-Natta-type polymerisation of ethylene and higher α-olefins. One of the most intriguing advancements in this field was the introduction of ansa-metallocenes or metallocenophanes which contain a bridging moiety between the two η5-coordinated fragments. These have allowed for the synthesis of a vast range of novel stereo regular polyolefins. Another great success was the development of related Group 4 constrained geometry complexes (CGCs) where one of the η5-coordinated ring systems of a metallocenophane is formally replaced by an η1-coordinated amido moiety. Due to their more accessible active site, these compounds display superior characteristics in the copolymerisation of ethylene and higher α-olefins, resulting in another class of polyolefins with exceptional features.
Both metallocenophanes and CGCs need to be activated for the polymerisation of olefins, with methylaluminoxane (MAO) and boron-based perfluorinated Lewis acids being the most common cocatalysts.
Why a boron bridge?
![]() Having these developments in mind, our group is interested in a replacement of the most commonly applied silicon bridge in metallocenophanes and CGCs by one containing boron. This bridging element may have potential Lewis acidic character, thus favourably altering the catalytic activity of the activated species and possibly allowing for self-activation. Furthermore, the comparably short and therefore rigid boron bridge should enhance selectivity of transformations catalysed by these complexes, e.g. the stereoregular polymerisation of propylene. In [2]borametallocenophanes, the reactivity of the diborane(4)diyl bridge may potentially be used for supporting the complexes or preparation of novel polymers via ring-opening oolymerisation (ROP).
Having these developments in mind, our group is interested in a replacement of the most commonly applied silicon bridge in metallocenophanes and CGCs by one containing boron. This bridging element may have potential Lewis acidic character, thus favourably altering the catalytic activity of the activated species and possibly allowing for self-activation. Furthermore, the comparably short and therefore rigid boron bridge should enhance selectivity of transformations catalysed by these complexes, e.g. the stereoregular polymerisation of propylene. In [2]borametallocenophanes, the reactivity of the diborane(4)diyl bridge may potentially be used for supporting the complexes or preparation of novel polymers via ring-opening oolymerisation (ROP).
Preparation
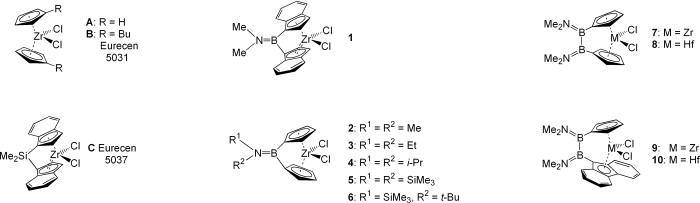
![]() A series of [n]borametallocenophanes (n=1, 2) of Ti, Zr and Hf with various ligand systems and related CGCs could be prepared in our group. Starting from the dihaloboranes the ligands have been synthesised by reaction with Na[C5H5] or Li[C13H9].
A series of [n]borametallocenophanes (n=1, 2) of Ti, Zr and Hf with various ligand systems and related CGCs could be prepared in our group. Starting from the dihaloboranes the ligands have been synthesised by reaction with Na[C5H5] or Li[C13H9].
[1]Borametallocenophanes
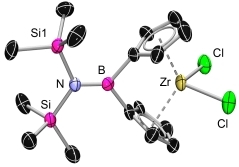
![]() The [1]borametallocenophanes could be prepared via salt elimination from the dilithiated ligands with group 4 metal halides, i.e. [TiCl3(thf)3], [ZrCl4(thf)2] and HfCl4 at low temperatures.[1, 2, 3]
The [1]borametallocenophanes could be prepared via salt elimination from the dilithiated ligands with group 4 metal halides, i.e. [TiCl3(thf)3], [ZrCl4(thf)2] and HfCl4 at low temperatures.[1, 2, 3]
[2]Borametallocenophanes
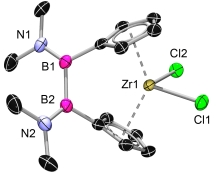
![]() The [2]borametallocenophanes can be prepared in the same manner like the [1]borametallocenophanes. The ligand can be synthesized from the dihaloborane with a excess of NaCp oder LiFlu, in arbitrary order. After deprotonation of the ligand with MeLi, the metallocenophane can be prepared via salt elimination.[4]
The [2]borametallocenophanes can be prepared in the same manner like the [1]borametallocenophanes. The ligand can be synthesized from the dihaloborane with a excess of NaCp oder LiFlu, in arbitrary order. After deprotonation of the ligand with MeLi, the metallocenophane can be prepared via salt elimination.[4]

Constrained geometry complexes
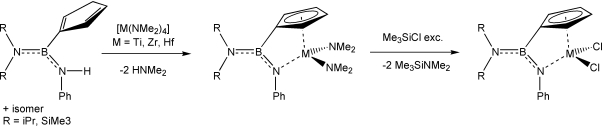
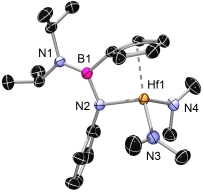
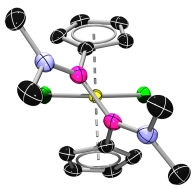
![]() The CGCs, where one of the η5-coordinated ring systems is formally replaced by a η1-coordinated amido moiety, were synthesised via amine elimination with [M(NMe2)4].[5, 6, 7]
The CGCs, where one of the η5-coordinated ring systems is formally replaced by a η1-coordinated amido moiety, were synthesised via amine elimination with [M(NMe2)4].[5, 6, 7]